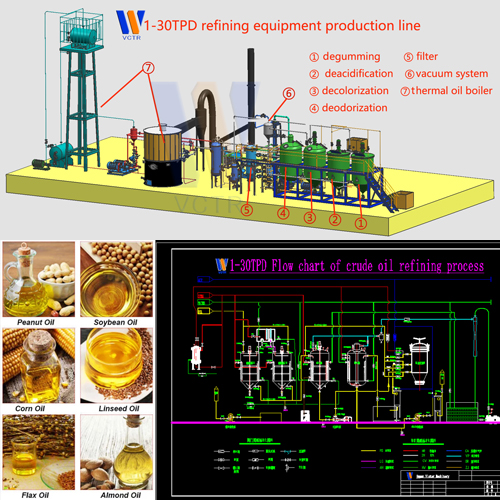
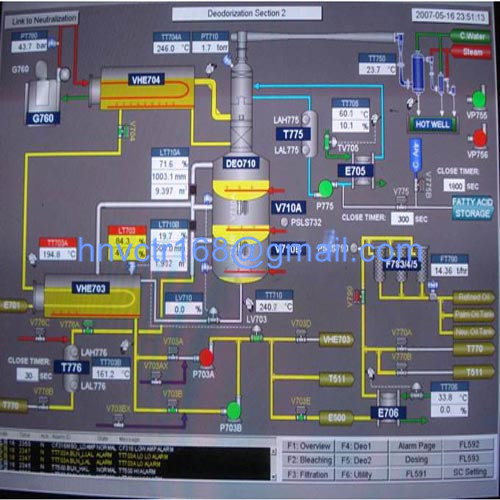
The source of pigment in vegetable oil
The pigments in vegetable oil are mainly derived from natural pigments, processed pigments, and storage pigments.
1. Natural pigments
The natural pigments in oils mainly include chlorophyll, lutein and carotene. Due to the existence of these pigments, the oils show different colors. For example, chlorophyll makes the oil green, lutein makes the oil yellow, and carotene makes the oil red. Most of these fat-soluble pigments enter the oil during the oil preparation process.
2. Processing pigments
Some pigments in oils are formed during oil processing. Oils and fats are affected by machinery, moisture, oxygen, and high temperature during processing. Proteins, sugars and other components undergo complex physical and chemical changes, resulting in polymerization, oxidation, and hydrolysis reactions to produce aldehydes, ketones, lower fatty acids, oxidative degradation products, Peroxides, epoxides and high molecular polymers. The content of phospholipids and metal ions in soybean oil is positively correlated with the coloring during storage and processing. Soybean phospholipids have poor oxidative stability and brown after oxidation. Metal ions are oxidation inducers. Metal ions mainly refer to Fe2+. Fe2+ is greater than or equal to 0.2 mg/kg, which promotes the oxidation of oils and produces processed pigments. Activity of metal ions Cu>Fe>Cr, Cb, Zn, Pb>Ca, Mg>Al. When the content of Cu and Fe ions exceed 0.01 mg/kg and 0.1 mg/kg, respectively, the oxidation of oil can be promoted. When the phosphorus content of soybean oil exceeds 25 mg/kg, the iron ion concentration will increase and the oxidation stability will deteriorate. High temperature and metal ions oxidize the tocopherol in the oil to produce dimers and tocopherol quinones. In soybean oil, the total amount of γ-tocopherol and γ-tocopherol dimer (γ-TED) is greater than 550 mg/kg, and no reversion and acidity will occur. Chlorophyll transforms into a red variant of lutein at high temperatures, free fatty acids (FFA) and iron ions produce dark iron soap. Residual phospholipids undergo pyrrolization of phospholipids after soybean oil deodorized (4,5-epoxy-2-heptenal reacts with multiple amino-containing phospholipids to produce polymer pyrrole phospholipids). This is a carbo-ammonia reaction that produces non-enzymatic Browning, causing color reversion.
3. Storage of pigments
Oils deteriorate during the storage period. Proteins, carbohydrates, phospholipids and colloidal hydrolyzed pigments are positively charged and suspended in the oil. When oil is stored for a long time, the impurities in the oil become crystal nuclei and form trace crystals. Grease comes into contact with iron in the storage tank, and the formation of oxides accelerates as the temperature rise. The color of the grease deepens with the prolonged storage time.
1. Natural pigments
The natural pigments in oils mainly include chlorophyll, lutein and carotene. Due to the existence of these pigments, the oils show different colors. For example, chlorophyll makes the oil green, lutein makes the oil yellow, and carotene makes the oil red. Most of these fat-soluble pigments enter the oil during the oil preparation process.
2. Processing pigments
Some pigments in oils are formed during oil processing. Oils and fats are affected by machinery, moisture, oxygen, and high temperature during processing. Proteins, sugars and other components undergo complex physical and chemical changes, resulting in polymerization, oxidation, and hydrolysis reactions to produce aldehydes, ketones, lower fatty acids, oxidative degradation products, Peroxides, epoxides and high molecular polymers. The content of phospholipids and metal ions in soybean oil is positively correlated with the coloring during storage and processing. Soybean phospholipids have poor oxidative stability and brown after oxidation. Metal ions are oxidation inducers. Metal ions mainly refer to Fe2+. Fe2+ is greater than or equal to 0.2 mg/kg, which promotes the oxidation of oils and produces processed pigments. Activity of metal ions Cu>Fe>Cr, Cb, Zn, Pb>Ca, Mg>Al. When the content of Cu and Fe ions exceed 0.01 mg/kg and 0.1 mg/kg, respectively, the oxidation of oil can be promoted. When the phosphorus content of soybean oil exceeds 25 mg/kg, the iron ion concentration will increase and the oxidation stability will deteriorate. High temperature and metal ions oxidize the tocopherol in the oil to produce dimers and tocopherol quinones. In soybean oil, the total amount of γ-tocopherol and γ-tocopherol dimer (γ-TED) is greater than 550 mg/kg, and no reversion and acidity will occur. Chlorophyll transforms into a red variant of lutein at high temperatures, free fatty acids (FFA) and iron ions produce dark iron soap. Residual phospholipids undergo pyrrolization of phospholipids after soybean oil deodorized (4,5-epoxy-2-heptenal reacts with multiple amino-containing phospholipids to produce polymer pyrrole phospholipids). This is a carbo-ammonia reaction that produces non-enzymatic Browning, causing color reversion.
3. Storage of pigments
Oils deteriorate during the storage period. Proteins, carbohydrates, phospholipids and colloidal hydrolyzed pigments are positively charged and suspended in the oil. When oil is stored for a long time, the impurities in the oil become crystal nuclei and form trace crystals. Grease comes into contact with iron in the storage tank, and the formation of oxides accelerates as the temperature rise. The color of the grease deepens with the prolonged storage time.